Part 3! Who developed quantum theory and why? What experiments motivated the creation of quantum mechanics? How was quantum mechanics a total break from classical physics? I discuss these questions and more in today’s Ask a Spaceman!
This episode is sponsored by BetterHelp. Give online therapy a try at betterhelp.com/spaceman and get on your way to being your best self. Visit BetterHelp to get 10% off your first month!
Join Paul's Audio Book Club on Chirp. There are no commitments or subscription required. Listen along with Paul, and you can enjoy deeply discounted prices (for a limited time) on each of Paul's Book Club Selections! Just go to https://www.chirpbooks.com/spaceman and follow the club to stay in the loop for future picks and other exclusive content.
Support the show: http://www.patreon.com/pmsutter
All episodes: http://www.AskASpaceman.com
Follow on Twitter: http://www.twitter.com/PaulMattSutter
Like on Facebook: http://www.facebook.com/PaulMattSutter
Watch on YouTube: http://www.youtube.com/PaulMSutter
Read a book: http://www.pmsutter/book
Go on an adventure: http://www.AstroTours.co
Keep those questions about space, science, astronomy, astrophysics, physics, and cosmology coming to #AskASpaceman for COMPLETE KNOWLEDGE OF TIME AND SPACE!
Big thanks to my top Patreon supporters this month: Justin G, Chris L, Barbara K, Duncan M, Corey D, Justin Z, Andrew F, Naila, Scott M, Rob H, Justin, Louis M, John W, Alexis, Erin J, Jennifer M, Gilbert M, Joshua, John S, Thomas D, Michael R, Simon G, David B, Frank T, Tim R, Tom Van S, Mark R, Alan B, Craig B, Richard K, Dave L, Stephen M, Maureen R, Stace J, Neil P, COTFM, Stephen S, Ken L, Alberto M, Matt C, Joe R, David P, Ulfert B, Sean M, Edward K, Darren W, Tracy F, Sarah K, Steven S, Ryan L, Ella F, Richard S, Sam R, Thomas K, James C, Jorg D, R Larche, Syamkumar M, John S, Fred S, Homer V, Mark D, Colin B, Bruce A, Steven M, Brent B, Bill E, Tim Z, Thomas W, Linda C, David W, Aissa F, Marc H, Avery P, Scott M, Thomas H, Farshad A, Matthias S, Kenneth D, Maureen R, Michael W, Scott W, David W, Neuterdude, Cha0sKami, Robert C, Robert B, Gary K, Stephen J, dhr18, Anna V, Johanna M, Matthew G, Paul & Giulia S, Ron D, Steven M, Louis M, Michael C, Alyssa K, Lode D, Roger, Bob C, Patti H, Red B, Benjamin M, BlueDragon, Stephen A, Ian S, James R, Skip M, Robert O, Adam I, and Lynn D!
Thanks to Cathy Rinella for editing.
Hosted by Paul M. Sutter, astrophysicist and the one and only Agent to the Stars (http://www.pmsutter.com).
All Episodes | Support | iTunes | Spotify | YouTube
EPISODE TRANSCRIPTION (AUTO-GENERATED)
Before I dig into today's topic, I wanted to share with you my latest recommendation in my Chirp audio book club. Chirp is a wonderful sponsor of this podcast and they're an audio book retailer known for their great deals without any commitment or subscription. And like I've done for the past few months, I'll announce a pick here and Chirp will deeply discount the audio book for a limited time. My pick this month is Our Universe by Joe Dunkley. I frankly, I wouldn't want to live anywhere else. I also literally couldn't. The universe is a big messy and wonderful place. And Joe Dungy's book is a fun engaging tour of all the crazies in the cosmos. I love this book for its breadth of subjects in the way that it highlights all the most common questions, questions that you have asked me. So if you want a fresh perspective and you're a little tired of hearing my voice, talk about all these wonderful things in the universe. Give this book a try. Join the audio book club, go to chirp books dot com slash spaceman and grab my first pick our universe on sale from $19 to 2 99 for a limited time and be sure to press the follow button to join my club and stay in the loop on future picks.
That's chirp books dot com slash spaceman going there really supports the show. Thank you. It turns out that the quantum nature of reality was there all along. We had just been missing it the whole time. Welcome to part three on this dive. Deep delicious dive into the wonderful subatomic world of quantum mechanics. In the last episode, I talked about the postulates the basic building blocks of the theory of quantum mechanics. And if you didn't listen to that, that episode, that's OK. You missed some pretty far out stuff. But don't worry, there's plenty more where that came from. And especially I wanted to focus on the theory of quantum mechanics as understood by physicists. You see a, a chemist, for example, have a different understanding of quantum mechanic. I've flipped through a lot of chemistry textbooks and they start with the Schroedinger Equation. And from there, go on to building uh electron orbitals and molecules and, and all the stuff relevant for chemistry and that's fine.
There's absolutely no problem with that. And that's a great starting point for quantum mechanics, but it's not the starting point that physicists use to understand the subatomic world. And because I am literally a physicist and I was trained in physics, uh I wanted to give you the physicist perspective and the physicists perspective is really abstract and just makes a bunch of vague statements about the world. And it's hard to connect those vague statements to the actual physical experience that we have either with our eyeballs or with our laboratory experiments. But that abstraction of the postulate their broadness and vagueness and how they weren't tied to any specific scenario and just made some very general statements about the the universe that that was also beautiful and wonderful because they completely capture the essence of subatomic life in just a handful of sentences.
Their abstractness gives them enormous power. Like I explained in the last episode, a handful of sentences that seem really vague and not pinned down to any specific scenario completely and totally described the entire subatomic world. But as we saw that abstractness makes them a little, you know, disconnected from reality. It's a little hard or let's be honest, very hard to connect those broad math statements with things that we can see and touch and smell or in the case of the subatomic world, what our experiments are telling us about what's going on down there. But once you have the postulates, once you have those seven statements, which are sometimes organized into four statements. Once you have those seven statements, you can build a cake, those are the ingredients, that's the flour, that's the butter, that's the sugar, that's the baking soda, that's the vanilla. The postulates are the ingredients and then you can mix them all together and you can build a cake that gives you a complete quantum theory, a quantum theory that can make predictions a quantum theory that can agree with experiment uh quantum theory that can explain a wide variety of experimental results.
So you start with those postulates and you work very hard. You're able to use the mathematics and logic and predict say the kind of light that will be emitted by hydrogen atoms or how electrons will behave when you shoot them through a thin metal screen. Or so many other subatomic experiments that we're beginning to perform at the start of the 20th century and continue to perform today. That said the reality of the creation of quantum mechanics was a little messier than that. It wasn't this clean look at these experiments. Let's develop a set of coherent postulates. And from there develop a a consistent theory of the subatomic world. No, no, no, that's not reality. That's never reality. What actually happened is we had a bunch of experiments that were starting to come online. And then there were a bunch of folks spitballing any random idea, any mathematical trick, anything that could possibly explain those results. Most of those ideas were wrong. Some of them were successful, some of them were partially successful.
Some of them were radically successful. Some of them made radical statements, some of them made less radical statements, some were less weird, some were more weird. They're just ideas. And then a collection of ideas began to coalesce and then that is what we now call quantum theory. Once we had the collection of ideas that could explain the quantum world, the subatomic world, then we went back and tried to put it on firmer mathematical grounds and develop a set of principled statements about the ever some ground truth about the subatomic world. Make sure everything was all logically consistent and hung together well and could explain all the results with no gaps, no overlaps and all that that came later, uh decades later. Actually, this is usually the case when it comes to physics. Usually we have a bunch of experiments or observations and we scramble to come up with anything that works and then slowly over time with a lot of argument, a picture emerges and then we go back and try to make that picture make sense.
Um the universe is messy and our attempts to understand it are messy too. But now that we're armed with the principles of quantum mechanics, like what I gave you in the last episode was modern quantum physics. This is the quantum mechanics that we understand it today that's taught today, that's in textbooks today that is applied today. But let's rewind the clock to see how the developers of the theory develop the theory. Let's see how they made the transition from classical physicists operating in a classical setting to quantum physicists operating in a quantum setting and I can't overemphasize just how big of a revolution this was and how quickly it came and went. We're going to open the 20th century with Max Plank's one weird trick to solving the black body problem. In just 25 years later, we're going to have a complete quantum theory, a quantum theory that flies in the face of the entire history of physics.
Up until that point, folks, it's like going from Mozart to Death Metal in the span of a couple decades with only a single generation of musicians responsible for bringing in that change. And those musicians were trained in Mozart. They started from Mozart at the beginning of their careers. And then at the end of their careers, they are inventing Death Metal. And then all future musicians will learn Mozart. But then we also learn Death Metal because Death Metal becomes such an integral part to music. Think of the differences between Mozart and Death Metal, the physicists that are going to develop quantum mechanics. They're like not just trained in Mozart. They are Mozart. Imagine going to school in classical music and then you go to a conservatory and then you're in an orchestra and you're perfecting your craft in classical music.
You're making arrangements and compositions and Sonatas and symphonies and, and all the stuff in between. And then halfway through your career, you start creating out of nowhere. Death metal. Imagine Mozart himself doing that. Imagine Mozart in one stage of his career creating the 29th symphony and then 20 years later, thrashing, that is what we're talking about. The transition from classical to quantum physics is like the transition from classical music to death metal in 25 years with a single generation of scientists building from nothing going directly from classical to death metal. Skipping all the between stuff, all the evolution skipping, jazz and R and B and rock skipping all of those and jumping right to death metal.
That is what we're talking about. The transition from the classical to the quantum understanding of reality was messy and complicated, but at least it was over and done with fast contrast that with the development of the atomic theory over 100 years to go from the first experimental signs of the existence of atoms to yeah, atoms do exist that took over 100 years. But then as soon as we decided and realized that atoms do exist and that sub atoms exist, it took us less than 25 years to develop a radical new theory of the physics of that world. So how did we go from Mozart to Death metal in the span of a single generation? And why was it so contentious? Well, there are many, many, many, many ways to approach the history of the development of quantum mechanics. There were a lot of people involved, there were a lot of ideas involved. There were a lot of arguments involved. It was messy and complicated. I'm gonna pick a particular path, this a path. I'm actually copying from a book.
The book is called Introductory Quantum Mechanics by Richard Lebov. Uh The introductory in the title is a little misleading. It's, it's a graduate textbook. It was my textbook in graduate school. When I learned the real deal of quantum mechanics. I want to bring this approach to this show because this approach closely matches what physicists consider the highlights. If you look back this like 20 to 30 year time period where we went from Mozart to death metal, from classical to quantum physics to get the story done in a single sitting, you need to just only cover the highlights and and these, these are the highlights that physicists seem important since I'm a physicist, it seems appropriate and those highlights are a series of experiments and theoretical insights that eventually give rise to quantum mechanics. Prior to the quantum revolution, the death metal revolution of the universe, there were some burning unanswered questions. For example, at the end of the 19 hundreds, we were beginning to get a sense of the wave particle nature of light.
And that's why I started this episode saying that the quantum nature of reality was there all along. We just had missed it because for centuries, physicists had debated the nature of light of whether it was wave based or particle based by the uh for a long time. We're talking like Newton who was assumed to be particle based. And then you get into the 18 hundreds and it appears more wavelike. And then finally, with Maxwell's equations, they're like, yeah, definitely waves. But then at the later end of the 18 hundreds, we started to get some more and more evidence that maybe there was some particle nature. So like the wave particle nature of light had been there all along. And that turned out to be our opening into the quantum world. Also, at the end of the 18 hundreds, we had discovered elements realized that they were Adams we had discovered spectroscopy. We had discovered that individual elements would have their own fingerprints of light emission that if I take a tank of argon or or hydrogen or whatever and and shoot it full of electricity, uh it glows but it doesn't glow with all wavelengths of light, only very specific wavelengths of light.
And this was a huge enduring mystery that was begging for a solution. This was one of the things motivating as we open the 19 hundreds, as we open the 20th century, we have two pillars. We have what is the nature of light? And where does spectroscopy come from? Why do atoms only emit certain kinds of light we start to develop in the early 20th century. Some more sophisticated experiments we begin to discover electrons, we begin to discover the atomic nucleus, we be begin to discover the nature of spin when it comes to subatomic particles. So we're trying to understand light in atoms. That is how we open the 20th century in the journey of quantum mechanics. Ali is highlighted in a physics textbook for instructing physicists starts with trying to understand light and ends with trying to understand atoms. And we, what we see is the importance of things we learn about light specifically wave particle duality and we import those ideas to understand how atoms work.
The there were two separate threads in the 18 hundreds. How do atoms work? What the heck is going on with spectroscopy? And then what the heck is going on with light? We start to learn some things with light, most importantly, the wave particle duality nature of light. And then we start to bring that idea into the world of atoms. And from there we get quantum mechanics in the solutions to these major unanswered problems. What is the nature of light? Where does spectroscopy come from? The solutions? Start with the man the myth, the legend Max Plank 19 01. Max Plank is studying something called black body radiation. Perhaps one of the most poorly worded setups in all of physics. It would it doesn't make any sense. I get it. Black body radiation is just OK. In the 18 hundreds, we developed this experimental device where we could put hot things inside of a chamber and then look at it into the chamber through a little opening. And then we were studying the light that was coming out.
So this was an experimental device to, to just try to understand light at a fundamental level. These devices were called black bodies. And then we were trying to understand the light. We couldn't understand the light that was coming out with everything we knew about the emission of radiation. Maxwell's equations, how particles or, or a atoms behaved and all that In the late 18 hundreds, we were getting the wrong answer. We could not explain the spectrum of radiation coming out of these little devices. All of our theories predicted way too much high energy radiation than was actually coming out. Max Plank. He came up with a way to solve this and correctly predict the kind of radiation that came out of these little devices through a a nasty hack in the equations. The nasty hack in his equation was that maybe radiation is emitted in chunks, maybe all the atoms and molecules and bits and pieces of my experimental device instead of being able to give off any kind of radiation, any kind of light possible, maybe it can only give off like certain amounts of light.
Like there's some fundamental chunkiness to light, maybe light is quantized, maybe there is a briefest dimmest possible flash of light that can ever be created at a certain energy level. And you can either have one of those flashes, two of those flashes, three of those flashes, four of those flashes added together. You can't have 1.5, you can have a quarter of one. You can have pi times flashes. You can't have that. You can only have one flash or twice that amount of flash or three times that amount of flash. And that did it, that did it, that solved the problem. Plank would end up winning the Nobel Prize for this discovery. He didn't really believe in the quantization of radiation, the quantization of light this whole time. He's like, no, no guys, I wasn't serious. It was just a little joke, a little, a little Plank prank or I like just chill out like there's a real answer and I don't know what the real answer is. I just introduced this nasty hack well, that it's been over 100 years and the nasty hack has not gone away.
He also discovered in his calculations that the energy and wavelength of a light are connected light of a specific wavelength or of a specific frequency has a specific amount of energy. So he found a very fundamental connection between energy and wavelength when it came to light. The next step, the next highlight comes in 19 05 with Albert Einstein. Albert Einstein was studying something called the photoelectric effect. It again when you, when you think about these experimental devices and what revealed the quantum nature of reality to us, they're wild. They're like, who would think of this, in the case of the photoelectric effect, it was hey, I wonder what would happen if we shined a light on a metal bar and made the electrons jump off? Like how fundamental is that? How basic is that? Who thinks that that kind of stuff? You know, bored physicist, that's who the photoelectric effect was. OK. Take a metal bar, shine a light on it. The metal bar has a bunch of electrons in it.
They're gonna get a bunch of energy from the light and they're gonna go flying off. So this is a great way to, to experiment and test and probe and study the connection between light and matter. And the results didn't make any sense. If the light had the wrong frequency, then no electrons would jump off of the metal regardless of the intensity, which didn't make sense in classical physics in classical physics is the frequency of the light shouldn't matter. You should just be able to keep pouring on light and eventually more and more energy you're adding tons and tons of energy to this metal bar. Eventually the electrons are just gonna get so agitated that they're gonna go flying off the metal. But they weren't. If you had the wrong frequency of light, the electrons would never jump off the metal no matter how many light bulbs you were shining on it. That was weird. Einstein figured it out. He made a leap based on Max Plank's results. So Max Plank said that OK, maybe radiation is emitted in chunks, maybe the emission, the actual glowing mechanism of light is quantized.
Einstein said maybe light itself is quantized. Maybe radiation is chunks. Eventually these chunks will get a name. They were called patreon patreon dot com slash PM. Sutter P MS U TT. Er It's how you can support the show. They were called photos. Einstein introduced the idea that yes, light acts like a wave but maybe it can have particle properties. And to explain the photoelectric effect. Einstein realized that the photons have to have the right frequency to get the electrons to jump off the metal because there's the electrons need a certain amount of energy to get off the metal. And only the right frequencies of light will have just the right energy to get the electrons to leave the you need the right frequency of light. So the light has the right energy that they can deliver to the electrons so they can escape from the metal. So you need the right key to open up the lock of the electrons in the metal. This result the photoelectric effect. His explanation for it was bang on dead accurate, explained it 100%.
It was the like the only way to explain the photoelectric effect. This was largely ignored by the community at the time. No one was eager to believe that light had particle properties and came in tiny little chunks called photons. But Einstein was a true believer. We'll see Einstein again and again, in this history of, of really being the one to get quantum mechanics started. He's behind the scenes, he's writing letters, he's encouraging people. Uh He's, he's, he's seeing further ahead. A lot of people say Einstein was the first modern physicist and that's not a bad way to characterize him. Another major ingredient that Einstein introduced was he in his analysis of the photoelectric effect. He introduced the idea of probabilities. He realized that when you're talking about atoms or electrons in a metal and photons and stuff that there needs to be a statistical treatment that you can only talk about averages and probabilities. I'm treating this very, very lightly, but I just want to highlight that, that Einstein was the first to introduce the idea of probabilities in the subatomic world.
Now, a word from our sponsor, better help. One of my favorite things about being a physicist is that the training I've received in physics is training to solve problems. It's training to look at difficult complex mind bending problems and find a simple solutions to, to take baby steps to find approximations and problem solving itself is a great skill that I found that physics has helped me with it. And you know what else can help with that? It's therapy. I I regularly speak with a therapist I've known and trusted this. The therapist for years was this person has guided me through very difficult points in my life and, and moments of, of easy sailing and just it's there someone who is close a confidant and who also understands people which was not a part of my physics training. If you're thinking of giving therapy a try, I want you to try better help.
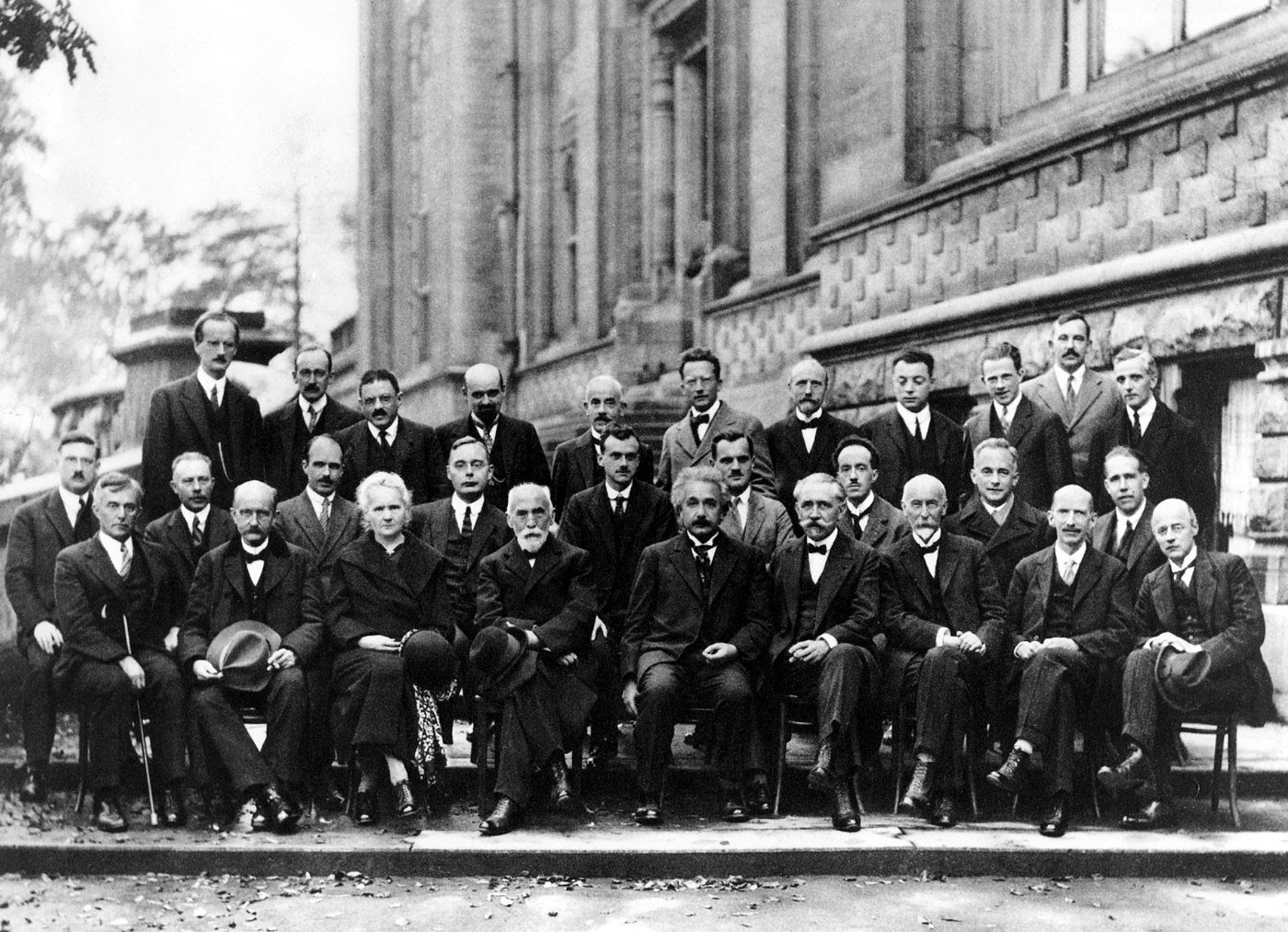
It's convenient, accessible, affordable, entirely online. Seriously. Give it a shot. I I can't advocate for mental health anymore when you want to be a better problem solver therapy can get you there visit better help dot com slash spaceman today to get 10% off your first month. That's better he LP dot com slash spaceman. The next step was 1913, Neil's bore develops the first quantized model of the atom. You know, Plank and Einstein were focused on the nature of light. Neil's Boer was focused on the nature of atoms and he was trying to explain spectroscopy. He was trying to explain why if I give you a hydrogen atom and it starts glowing. The kind of light that comes off of it is at only very specific wavelengths, there's a wavelength here and the emission at another wavelength. And another one, you don't just get this broad continuous spectrum of all sorts of wavelengths. The emission of light from atoms appeared to be quantized.
He came up with a quantum the atom. He said maybe electrons can only be in certain orbits. This was a very appealing model because everyone can visualize a tiny little solar system instead of a sun, you have an a nucleus, instead of planets, you have electrons. It's very easy to think about, very easy to visualize. And it just said, bore himself said, I don't know why the electrons are confined to these very specific orbits. I just know that they are and it's this orbit in this orbit in this orbit. And then when they change from one orbit to another, that's when you get the emission of radiation. And because they're changing from one orbit to another, that's a very specific amount of energy that is released, which means you get one photon associated with that with a very specific wavelength. So he's tying back to Max Plank's idea that the energy of light, the energy of a photon is related to the wavelength of the photon. He's tying back to Einstein's idea that light itself is quantized. And he's saying, hey, if light can be quantized, maybe atoms could be quantized too, maybe.
And the electrons in energy levels in an atom are tied, you know, it's like a hotel, you can be on the first floor or the second floor or the third floor, you can't be in between, there are no rooms there. And if the electron goes from the third floor to the second floor, it's gonna release some energy in the form of a very specific frequency of light. Also introduce some randomness to the idea or start to introduce the concept of randomness when it came to atoms. Because he, he, he realized that you don't know when an atom will emit a photon. It's just sitting there, eventually, it will emit a photon. Eventually it will give off some light. But you don't know when you can't say when the atom is gonna do it. So we're starting to see by 1913, it becomes apparent that the subatomic world is playing by different rules. Light acts as both waves and particles, not every option is available. Some things are quantized in randomness and probabilities start to play an important role.
At this point. In the story, everyone needs to pause because of world war one, which is a bad time to be doing physics. After the war, physicists start looking at this quantum theory again which had some interesting ideas but was not complete. Neils Bo couldn't explain why the electrons had very specific orbits. No one was really sure if Einstein was right and radiation light really was quantized or not in 1924 someone makes a breakthrough that someone was a phd student, Louis Deroy. Now the name, the last name is spelled as French, it's spelled and then Broy is Boglie. So of course, every physicist says de broccoli because it sounds funnier. So Louis de Broccoli in his phd thesis said, look, Einstein's over here talking about light being both a wave and a particle like sometimes the wave nature comes out and sometimes the particle nature comes out but it can be both.
So if light can do it and light is quantized and we see that electrons are quantized in an atom according to Neil spore, maybe matter itself has both wave and particle natures. Light's wavelength is tied to its energy, right? The energy of a photon tells you exactly what its wavelength is. That's plank figured that out in 19 01. And if I take an electron or a baseball or myself and I start moving, I have kinetic energy, I'm moving, I have energy too. And so maybe I have a wavelength associated with me through a similar relation. If I can relate the wavelength of light to the energy of light, maybe I can relate to the energy of matter to the wavelength of matter. His advisor wasn't sure if this idea, this hypothesis was genius or crazy. So he wrote, Einstein and Einstein wrote back and this is probably the most momentous sentence in the development of quantum mechanics in 1924 when Einstein replied, and in the letter Einstein said, quote, I believe that it involves more than a mere analogy.
So he's saying De Bro's idea to broccoli's idea wasn't just a mere analogy. Now, Einstein believed he was on to something eventually Deroy would win the Nobel Prize for this insight. What made De Bro's idea especially intriguing was that it provided an explanation for Boer's model. BR said, OK, the electrons in an atom have to be on certain floors. They can be in the uh this orbit, the second orbit, the third orbit. And these orbits are in very specific locations. And I can figure out what those locations are because the differences between those energy levels give us those very specific fr of light that we emit. So we know where the electron orbitals are, we know where those orbital distances are. But like why those and why not other ones? Why couldn't the first floor in an atom be a little bit closer to the nucleus or a little bit further? Why was it exactly here de Bro's hypothesis was able to provide an explanation because he said, look if an electron is also a wave or has a wave like nature and you're putting a wave in a circle around a nucleus, you can't have any wavelength you want, you, you have to have a wavelength that fits if you start drawing in a circle, a wave with a specific wavelength and you're drawing the, drawing the wavelength up down, up down, imagine drawing in a circle this, up, down, up down, up down.
When you come back to where you start, it has to meet up. If you get the wavelength wrong, it won't meet up, you'll, you'll be coming down when it's supposed to be going up, it won't fit and it's not allowed. It's just like the standing waves on a string or in a musical instrument, only certain wavelengths are allowed because they have to fit. And de Broy said, well, the electrons are at those orbitals, the electrons are at those distances away from the nucleus because that's the only places where their wavelengths can fit a lot of discussion. But what about what exactly matter waves are? De Bro's hypothesis was intriguing. It was tantalizing provided a hint that there might be something going on in the subatomic world that involves wave particle duality. But what are matter waves like we know what waves of photons are or waves of light are? It's electromagnetism, we already know that. And then they also have this like particle nature that we call photons.
And if you get a bunch of photons together, they stop looking like particles and they act like waves. OK. It but I can get a bunch of electrons together and they don't do that. So what is the wave nature of an electron? What is the wave nature of matter? What is the wave nature of you? And me, an electron is very obviously a particle. But what is the wave part? We see a split here and this was happening very, very quickly. There was one camp led by people like Neil Spore Max Bone Werner Heisenberg and others who said, forget it. Duroy, you're interesting. That's a cool idea. It's very cute but stop trying to interpret what's going on and just focus on what we can observe. What can we observe? We have no idea what's happening inside of an atom. We can't picture it's literally an atom. It's too small to, we can't get our cameras out. We can't interview it. Stop trying to treat subatomic physics like normal physics, like classical physics, classical physics, we can talk about and visualize and poke at and prod and look at the objects that we're studying.
We can't do that with subatomic physics. So stop trying, stop trying to interpret, focus on the spectra emitted by atoms. That's what we can observe. Folks, we have no idea what an electron is doing down there. We have no idea what an atom is really doing. What we have is the radiation that they emit. That's what we can see. Ignore the wave deroy is off his rocker. He may be right, he may be wrong. But it doesn't matter that this approach of Heisenberg born and born. It doesn't matter if Deroy is right or wrong. What matters is what we can observe in 1925. Just a year after de Bro's hypothesis, Heisenberg develops a complete mathematical description of subatomic processes. It is the first invention of quantum mechanics from his development. A couple years later, he would discover the uncertainty principle. Remember at this time, there are no postulates, there's no firm basis. Everything I described in the last episode is the quantum mechanics that we have today in 1925 we didn't have the postulates at the time Heisenberg was just trying to explain atomic spectra.
He's looking at anything that could explain why atoms have spectra, why the radiation that comes out of atoms is at very specific wavelengths and not any kind of wavelength you want. He was trying to explain the bore model of the atom and extend it to other atoms, other elements. He was trying to figure it out this creation of quantum mechanics due to Heisenberg didn't include anything about waves and instead use a, used a different set of mathematics known as matrices. Matrices are just uh a two dimensional arrays of numbers. If you've ever used a spreadsheet with rows and columns, you've used a matrix. The mathematics that Heisenberg used to understand atomic spectra and what was happening at a subatomic level was like an algebra version of spreadsheets at the time. This was before spreadsheets. This kind of math was relatively new and it wasn't well known by physicists. In fact, Heisenberg invented all the mathematics and then his advisor Neil SBO said, oh yeah, like I talked to a mathematician.
This looks familiar, I think these are called matrices. Like he accidentally involve invented matrices which mathematicians had already invented previously, but Heisenberg didn't know it, no one knew it. So most physicists didn't know what to make of Heisenberg's result. It did successfully predict pretty much all atomic spectra. So it was a huge success. He could explain the kind of light and predict the kind of light emitted by a hydrogen atom in a helium atom in oxygen and carbon. He could explain it all this approach though, had no picture whatsoever of the subatomic world. So it like took the bore model with this tiny little nucleus and little electrons over ground and just like put it to the side and said, boom, here you go. You wanna calculate, you wanna predict atomic spectra. Here's how you do it. Remember. Heisenberg is just guessing here. A lot of people were just guessing here, but Heisenberg nailed it. He figured it out. He developed the first functioning quantum theory working in parallel to all that was another camp with figures like Einstein and Irwin Schroedinger who were trying to interpret and understand what matter waves really were.
They really liked de Bro's hypothesis. They really liked this idea. They really liked this particle wave dual nature of reality and were trying to ask like OK, what is a matter wave? The Heisenberg camp said, forget it, let's just develop equations that help us make predictions. And then the Schrodinger camp was saying, no, no, no. Hold on. We we should make prediction. We we should have a picture of what's going on. Schroedinger develops a complete theory of quantum mechanics in parallel to Heisenberg. Heisenberg was first but then Schrodinger comes out in his theory is based on waves of the famous Schroedinger equation. It is an equation describing the evolution of matter waves of electrons, which was just Newton's laws applied in a very crazy situation. But he said, OK, if an electron has a wave nature, how does this wave nature evolve? And he was able to develop a theory of quantum mechanics since physicists were already familiar with the mathematics of ways, we've been dealing with ways for like centuries. This caught on much faster and much more widely than Heisenberg's approach.
They didn't really know what to do with Heisenberg's approach, but they knew what to do with Schrodinger's approach. It was naturally more intuitive to the physicists at the time because they had worked with very similar math. Uh They hadn't worked with matrices before. They weren't familiar with spreadsheets, but they knew a wave later that year in 1925 Schroedinger would demonstrate that both approaches were equivalent. You could actually translate from Heisenberg's approach to Schrodinger's approach and vice versa. For various math reasons that I'm not going to get into. Nowadays, this connection is obvious to mathematicians and physicists. But to them, it was strange and new. If you've already encountered quantum mechanics, you probably heard the phrase the wave function. This is where we get the term wave function. It's from the Schrodinger equation from his approach. I've been deliberately trying to avoid using the word way function in this series. It's a little overplayed, not the most important part of quantum mechanics, at least to a physicist to chemists uh the way function, the shorter equation is is key to what they do. But now to a physicist in the discussion of interpretations that we're gonna get into later, it is easier to digest if you just relax about all the wave stuff.
Yes, wave particle duality is a fundamental component of the subatomic world. We are gonna encounter arguments about what exactly the wave is. It's better to have an understanding of quantum mechanics from the math first, before we get into the wordy bits. And you should know because this debate, this split between Heisenberg and Schwinger is just gonna grow, we'll see this in future episodes. And then in many ways, it is the ground of the present arguments over the interpretation of quantum mechanics. It starts here with the fact that two people working in parallel completely independently come up with two completely different views of quantum mechanics. One is based on trying to understand what the wave nature of subatomic particles are and the other completely ignores it and just focuses on observations. It turns out that these two approaches are equivalent, give the same answers and you can translate from one to the other. That's how we why we have headaches the present day.
Even though the Schrodinger equation worked and made predictions, it wasn't exactly clear still what was waving like. Yes. Now we can follow the time evolution of the waves of these waves of electrons in an atom. What are they? It will be Max Bourne who would give the first interpretation of the wave function that allows calculations to be made with shorter's equation. Bourne was working on a different problem. He wasn't looking at atoms, he was working on something on scattering problems. Uh um If you take an electron and shoot it at a metal screen, it will get deflected it like a little change in its trajectory. Schroeder's equation was a wave equation. It talked about the propagation of this matter wave through the screen and it made it was correct. But at the end of the day, only one electron hits a screen, not a wave doesn't slosh up against your detector device, one electron slams into it. So you have to make a translation from wave to particle bone was the one to say what's waving is the probabilities that observations change the thing you're looking at.
And you can use the schrodinger equation to predict the probabilities of what you're about to observe. And then once you make the observation, the electron actually shows up somewhere all of this about probabilities and predictions and all that also appear in Heisenberg's matrix version of quantum mechanics. It's just a little less obvious. So people didn't see it right away, but it's all right there. That interpretation of what a w schrodinger matter wave is what the deroy wave is, is what I suspect the first interpretation of quantum mechanics. I'm deliberately not going into the details too much because that's the point where everything goes off the rails and triggers the debates that last into the present day. But we want to place a bookmark there to least say that it happened. And we'll, we'll, we'll come back to it. Don't worry. By the end of 1925 we have all the puzzle pieces of a working in quantum theory. But at this point in time, it's very sketchy, very shaky and poorly understood. We now know that there's like a wave particle nature of subatomic objects that we need to take account of an alternative explanation based solely on matrices that ignores the way particles stuff uh tells us about fundamental uncertainty.
And now both approaches, we realize that probability plays a major role in that the act of observation selects a possible answer. Some of these statements are more obvious in schroeder's approach, like the role of probability. While others are more obvious in Heisenberg's approach, like fundamental uncertainty. This fact that there are two approaches isn't so crazy in physics is actually very common to switch math because something sometimes the thing you're interested in is more immediate or obvious to see from a different approach with a different set of equations. Like it's easier to say some things in one language than another. It's a good thing I did that whole episode on on Lagrangian mechanics and the principle of least action because that's a perfect highlight, you can just switch languages and, and some things are more apparent what is crazy. However, is how these two approaches had completely different philosophies to approaching quantum mechanics. And how even though they could translate from one to another, it, it it wasn't immediately clear how they could possibly say the same thing. Schrodinger's approach was based on trying to understand the wave nature of matter.
Heisenberg's approach was purely based on just trying to find anything, any description that could predict the results of experiments without trying to worry too much about what was quote actually going on in an atom. How did these insights and equations connect back to the postulates? Well, it's not exactly a straight line to start. We had a list of experiments black body radiation photoelectric effect, atomic spectra, the scattering of subatomic particles. And we were trying to develop a coherent theory that described all subatomic behavior. Some attempts like Heisenberg's just focused on results without trying to build pictures of the subatomic world. Others like Schroedinger took what we knew from classical physics and try to import them into the subatomic world like oh waves, we know how to handle waves. Let's try to apply that. Heisenberg was like, let's invent something new. It started with half measures the bore model, the idea of photons debro matter waves and ended with Heisenberg's matrix mechanics and Schrodinger's wave mechanics, we had a working theory.
We had two working theories, but nobody knew what the theory meant. The next step was to put these vague solutions that were working, but we didn't understand and put them on for firmer ground, develop the first principles right down the postulates. It would take another decade. It would take until the 19 thirties when genius and polymath John von Neumann would take all the math Schroedinger Heisenberg's uh who is not appearing much in this series, uh developed his own way to approach quantum mechanics. We didn't even weren't even talking about that. Streamlined it into a set of principles which are the postulates that I talked about last time, which is the modern physicist view of quantum mechanics. So from there we get the mathematical bones that we can start working out the theory and it ends up being an incredibly successful theory of nature. But the seeds that were planted in 1925 persist, what is going on in the subatomic world? And how are we supposed to understand that? Well, that's the topic for another day. Thanks to Mihail E on email at Sharman on Twitter, Massimiliano, S on Facebook Isaac P on email at at Twitter, Chris F on Facebook, Akansha B on email at SMTR on Twitter, Albert R on email Julius, M on email Martin, E on email John T at Facebook Rice, C on email Nick on email Jordie R on Twitter at P.
It's a larger at Twitter, email HP, Arian and email Scott M on email, Graeme Dion, email Martin N on email at sample Sapiens on Twitter, Peter W on email at Mark Group on Twitter. Sean on email, Susan S on email. Daniel Jan, email Campbell Dion, email Timothy be on youtube Fernando Gian email and James W on email. Of course, you can keep contributing to the show dot com slash PM Sutter. I really do appreciate it and of course, I have to thank my top Patreon contributors this month, Justin GG, Chris L Barbeque Duncan, M Coy D, Justin Znh and F Naia Scott M Rob H, Justin Lewis, M Paul G, Don John W Alexis, Aaron J, Jennifer M Gilbert M Joshua Bob H, John S Thomas Dmr and Simon G. That's Patreon dot com slash PM Sutter. I really truly do appreciate it. Send me more questions. That's hashtag ask a spaceman. You can find me on social at Paul Mats Sutter, ask us spaceman at gmail dot com or just the website, ask us spaceman dot com and I will see you next time for more complete knowledge of time and space.
The, it's always the right time deal. Hey, you wanna go to Mickey D's for lunch? Oh, let's go now. But it's not lunchtime yet. If we're going to mcdonald's, it's always the right time. It's hard to argue with that. There's a deal for every lunch hour at mcdonald's. Now's the time to get two for 3 99 mix and match a four piece mcnuggets, a mcdole A mcchicken or a hot and spicy mcchicken. Price of participation may vary, cannot be combined with any other offer, a single item at regular price.